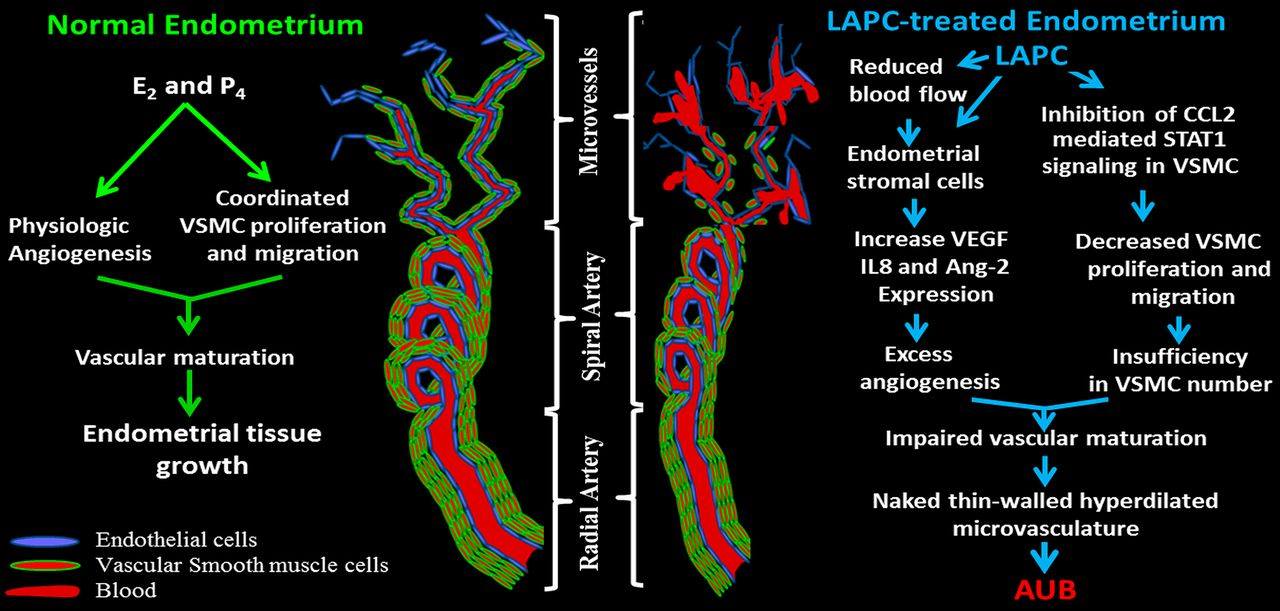
Model of aberrant vascular transformation and related AUB in women administered LAPCs. In normal endometrium, estradiol (E2) and progesterone (P4) regulate angiogenesis as well as VSMC proliferation and migration. Coordination of these steroid-mediated effects triggers vascular maturation resulting in a tight and continuous layer of VSMCs lying beneath endothelial cells of new vessels, which provides normal blood flow to induce endometrial growth. In contrast, long-term effects on human endometrium by synthetic progestins elicit: (i) reduced blood flow which induces local hypoxia; (ii) induced decidualization and increased expression of such angiogenic factors as VEGF, IL8, and Ang-2 in stromal cells; and (iii) inhibited VSMC proliferation and migration by blocking CCL2-mediated STAT1 signaling. The first two mechanisms promote excess angiogenesis, whereas the third mechanism results in insufficient VSMC numbers to cover and surround newly formed vessels. Consequently, impaired vascular maturation generates thin-walled hyperdilated fragile vessels which are prone to leakage and AUB.