Renal calculi can be managed according to four treatment options: conservative management, extracorporeal shock wave lithotripsy (ESWL), flexible ureterorenoscopy (FURS) and percutaneous nephrolithotomy (PCNL). Having addressed conservative management and ESWL in the last edition of Urology News , the second article in this three-part series will discuss FURS divided into the same sections used in the previous article:
- Patient selection (including ‘the perfect case’ and ‘the case to avoid’)
- Intraoperative decision-making
- Postoperative management / follow-up.
FURS
Patient selection
The EAU guidelines recommend ESWL as first-line treatment for renal calculi less than 10mm, and PCNL for stones more than 20mm in size. In the ‘grey zone’ between 10 and 20mm, FURS is recommended over ESWL in patients with challenging intra-renal anatomy, ‘hard’ stones, or with an unfavourable skin-to-stone distance, that would make ESWL less likely to be effective [1].
The perfect case
The ideal case for FURS is therefore a stone less than 15mm in diameter, in a patient with a normal lower tract with straightforward intra-renal anatomy (i.e. a short, wide, flat-angled infundibulum for access to any lower pole stones). As stones enlarge beyond 15mm in maximum diameter, and certainly beyond 20mm, the time required to disintegrate the stone to dust, or the number of passages of the scope needed to retrieve the pieces of a ‘fragment and extract’ strategy means that PCNL becomes the more favourable choice, particularly if the priority is achieving a stone-free kidney in a single procedure.
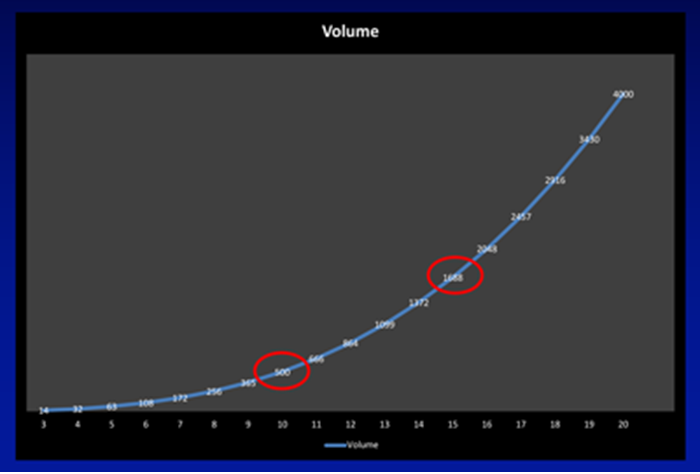
Figure 1a.
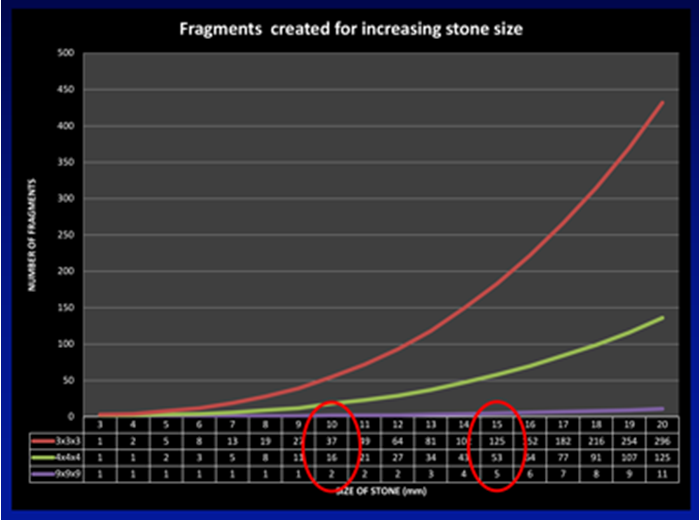
Figure 1b.
This can be demonstrated from the curves shown in Figure 1a and b. Assuming the stone is a sphere with its diameter increasing in 1mm increments on the X axis, according to the formula for an ellipsoid (volume = π x L x W x D x 0.167, which can be approximated to the diameter cubed and divided by two for day-to-day practice) its volume increases exponentially, such that a stone that doubles in diameter from 10 to 20mm has an eight-fold increase in its volume. Figure 1b shows the implications of this increase in volume in terms of the number of fragments stones of increasing size are comprised of. Assuming no disintegration of the stone during fragmentation, a stone of 10mm would generate 37 three-millimetre fragments, increasing to 125 fragments for a 15mm stone, and just under 300 fragments of this size from a 20mm stone.
“A stone that doubles in diameter from 10 to 20mm has an eight-fold increase in its volume.”
The mathematics helps explain the transition from ESWL for stones less than 10mm (where a sufficiently small number of fragments could pass spontaneously) through FURS for stones between 10-20mm, to PCNL for stones of 20mm and beyond (where spontaneous passage of nearly 300 three-millimetre fragments, or retrieval of 125 four-millimetre fragments via an access sheath make ESWL and FURS less attractive).
These calculations are supported by ‘real-life’ practice, and demonstrated in the CROES data, based on 1210 consecutive patients with a solitary kidney stone treated across 114 centres worldwide, with the key finding being that increasing stone size was positively correlated with operative time and negatively correlated with stone-free rate. Specifically, this study showed that the best results for single-session flexible ureterorenoscopy were obtained for stones less than 15mm. Stones less than 10mm in size had a 90% single-session stone-free rate, decreasing to just 30% stone-free rate for stones >20mm. This study showed an increased likelihood of postoperative fever (but not an increased risk of SIRS or sepsis), and risk of needing re-admission and retreatment in stones >20mm [2].
FURS also offers the option of bilateral stone treatment (see intraoperative decision-making), and has been shown to achieve an overall stone-free rate (SFR) of 88.6%, understandably better in smaller total stone burdens (100% for a stone burden <25mm compared with 80% for a stone burden ≥25mm [3].
Although meta-analysis data shows that PCNL has higher stone-free rates than FURS, this is at the expense of higher complication rates, blood loss, and a longer admission time [4]. Whilst the ‘battle lines’ between FURS and miniaturised versions of PCNL for stones between 15-20mm are being established, it is reasonable to continue to consider FURS as standard therapy for stones <2cm.
The case to avoid
Whilst larger stones can be managed by FURS, the patient should accept that this is likely to be a ‘multi-phased’ procedure with an interval JJ stent until the treatment has been successfully completed. This is exemplified by the findings of Riley et al ., who showed an overall stone-free rate of 90.9% for patients with a stone size up to 30mm, with a correspondingly long operation time of up to 138 minutes, and a median of two procedures per patient [5].
It is also possible that a third operation will be needed, and there is a risk of failure to treat the stone at all, particularly in the lower pole, where basket relocation may not be feasible if the stone is wider than the infundibulum, and therefore fragmentation in situ will be needed, at least to start with. This requires full deflection of the scope, which may be difficult, particularly with a laser fibre deployed. As already evident from the mathematics, large (>20mm) lower pole stones are therefore likely to be better treated by PCNL.
Intraoperative decision-making
Modern laser machines allow variation of the pulse frequency (from 4Hz to as high as 80Hz on some machines) and the energy (as low as 0.2J on some machines up to 2.0J) per pulse.
Lower pulse energy creates smaller fragments and less stone retropulsion, whereas higher pulse energy is associated with larger fragment size and with a degree of retropulsion. Some devices also allow the pulse width to be changed from a standard 300 to 350 microseconds to a longer laser pulse duration of between 700 to 1500 microseconds. In vitro studies have shown that the longer pulse duration, whereby the same laser energy per pulse is delivered over a longer period, allows effective stone treatment with less retropulsion and reduced degradation of the fibre tip. A long pulse width is therefore the ideal to complement a ‘dusting’ setting, whereas a short pulse width is more suited to a fragmentation strategy.
In general, one might expect a higher stone-free rate for basketing compared to dusting, but with a longer operation time and in-theatre costs (a basket / retrieval device, and often an access sheath to facilitate multiple scope passage for multiple fragment removal).
Fragmenting vs. dusting
Fragmenting – high energy / low rate / short pulse width
In vitro studies have shown that the use of a low frequency (5Hz) and high pulse energy (1.2J) created deeper and wider stone fissures in a stone model, and concluded that such settings offered the most efficient method for stone fragmentation [6]. In a clinical setting, if pure fragmentation is the goal, the middle of the stone should be targeted (with the added advantage of less urothelial trauma from the laser during respiratory movement), aiming to cleave the stone into two fragments, which can then be similarly targeted at their midpoint to create an increasing number of smaller fragments until they are sufficiently small to be retrieved with a basket. This judgement on stone fragment size is based on comparison of the fragments against landmarks such as the laser fibre itself (275 micrometers or so), a safety wire (1mm diameter) or against the tip of a papilla. Their behaviour with irrigation / flush of saline – ‘dancing fragments’ or ‘washing dust’ – is also useful. Finally, the exact appearance and size of the fragments can be examined when they have been removed.
The goal of the ‘fragment and extract’ approach is to remove all of the fragments with a basket, such that the patient does not need to pass fragments to achieve a genuinely stone-free kidney. This approach also provides a stone sample for biochemical analysis, which might be useful in directing the patient’s future medical preventative therapy. Compared with ‘dusting’, this technique is associated with higher immediate costs, both through the cost of the basket / extraction device, the fact that an access sheath will often be used to facilitate repeat instrumentation for fragment removal, and the tendency for the operation to take longer than one in which the stone is dusted. However, this must be weighed up against the longer term costs including the reduced need for additional treatment if there are no residual fragments to grow or cause future symptoms.
Dusting – low energy / high rate / long pulse width
The goal of dusting is to fragment the stone into tiny pieces that resemble ‘dust’ and thus can pass spontaneously. This is best achieved at a low energy / high frequency setting (using a long pulse-width if available), keeping the laser fibre slightly off the stone to ‘defocus’ it. Defocusing the laser minimises the mechanical effect of the laser energy and therefore produces smaller fragments (i.e. dust). The ideal technique is to ‘paint’ the stone from the edge toward the middle of the stone, generating tiny particles that are too small for basket retrieval, and will therefore pass spontaneously without incident.
This technique is associated with less need for an access sheath, and therefore less risk of ureteric trauma. Indeed, if the procedure has been straightforward, the avoidance of an access sheath means the ureter has not been dilated, and it may be possible to avoid a postoperative JJ stent. Omitting the additional steps of access sheath insertion, and particularly of repeated scope reinsertion to grasp and retrieve fragments means that dusting has been associated with a 20-40% reduction in operation time compared to the fragment and extract approach [7]. There is also the advantage that fewer instruments (primarily no basket) have to be passed through the ureterorenoscope’s working channel, and therefore may be associated with prolonged life of the scope.
However, some unquantifiable costs include the possibility of requiring future procedures for symptomatic or enlarging residual fragments, and the cost of emergency visits to hospital with ureteric colic for the passage of unexpected large fragments.
Popcorn – high energy / high rate / short pulse width
The ‘popcorn’ effect is used as an adjunct to fragmentation, allowing stone fragments to be ‘polished’ in a calyx, in which the laser fibre is deployed in the centre of the calyx using a high energy at a high rate, which allows the stone to be treated each time they ‘bounce’ into the immediate vicinity of the continuously activated laser tip.
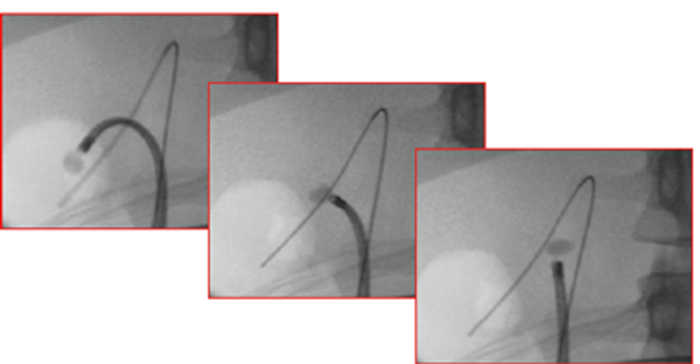
Figure 2. FURS relocation. These three images show a lower pole stone that has been secured in a basket and moved to the upper pole. The advantage is a straighter scope for treating the stone, avoiding the use of an activated laser fibre when the scope is deflected, which can risk fracture of the fibre with resultant laser damage to the scope. It is also more ergonomic to treat stones with the scope (and therefore surgeon’s wrist) straight.
In reality, the endoluminal endourologist needs to be proficient at all these techniques, as the best approach for an individual patient depends not only on stone size and location, but also its density (some hard stones are not suitable for dusting) and the ureteric and intra-renal anatomy of the patient. Sometimes a combination of the techniques will deliver the best results: for example, a high energy low rate setting might be needed to fragment a lower pole stone before it can be relocated and ‘dusted’ in a more convenient upper pole position (Figure 2). Alternatively, reducing a large upper pole stone into a smaller size by a low energy high rate ‘dusting’ setting before fragmenting and extracting the remnants may offer a good compromise between stone clearance, fragment analysis without having to extract multiple fragments.
Access sheath or not?
In tandem with the stone treatment technique is the decision whether or not to use a ureteric access sheath (UAS). This choice continues to divide endourological opinion: CROES data shows that approximately half of endourologists use them ‘routinely’ (i.e. in more than 80% of cases) whereas a substantial proportion (just under 20% of endourologists) never use one [8].
The decision behind this depends on the ureteric anatomy and the planned treatment strategy for the stone (i.e. dusting vs. fragmenting and extracting) and has a number of potential advantages for the case (Table 1).
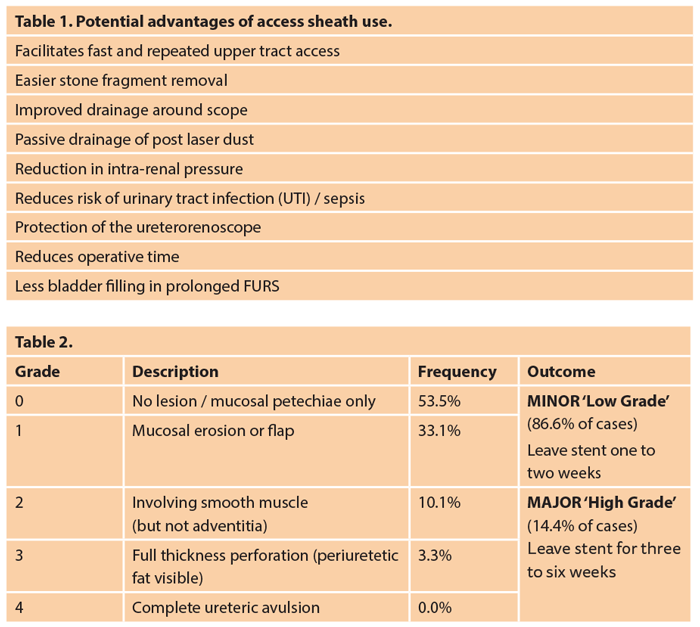
These potential advantages must be balanced against the cost of the sheath, not merely the expense of the consumable, but the risk of ureteric injury as classified by Traxer and Thomas [9]. This study identified ureteric wall injuries in 46.5% of patients, although the majority were classified as ‘low grade’ (and were managed by short-term stent insertion – Table 2); 13.4% had ‘high grade’ injuries, with the majority of these being grade 2 (i.e. preserving the ureteric adventita, such that extra-ureteric fat was not seen). The higher grade injuries were also successfully managed by JJ stent insertion using a longer dwell time of three to six weeks. The risk factors for a more severe injury included older age, male gender and the absence of a prior JJ stent. The latter seems to be the key risk reducing factor, with a seven times reduced risk of a severe injury if pre-stented.
The CROES study for access sheath use demonstrated that patients in whom UAS were used had higher preoperative American Society of Anaesthesiologists (ASA) scores, larger stones and longer operation times. The paper reports calculations based on regression analyses as to the benefit of access sheaths for stone clearance, but, as expected, raw data for the advantage of an access sheath in terms of stone-free rates was more noticeable in larger stones (48.1% vs. 34.2% for stones >10mm) than in smaller ones (51.9% vs. 65.8% for stones <10mm).
Reassuringly, this large study, based on 2239 patients, found no increased risk of intraoperative complications such as ureteral damage or bleeding from the use of an access sheath (a 1.1% ureteric perforation rate with an access sheath compared to 1.2% without). As would be expected, there was a tendency for an increased likelihood of a JJ stent following access sheath use (90.6% of patients versus 82.0% in whom a sheath was not used). The article noted that postoperative infectious complications were reduced in patients in whom an access sheath had been used, attributable to reduced intra-renal pressure in these patients [8].
As mentioned above in the section on patient selection, FURS offers the opportunity to treat bilateral renal stones at the same sitting. This is not feasible for ESWL due to to the risk of bilateral ureteric colic with acute kidney injury, and, although possible, a same-sitting bilateral PCNL is a substantial intervention. Bilateral FURS may avoid a second operation altogether (if the stone burden on both sides is small), but is more likely to be performed as part of a planned, staged procedure, with one side having a larger stone burden than the other. In general, the symptomatic side should be tackled first, but if the patient is equally symptomatic on both sides, the side with the greater volume of stone(s) should be tackled first. This allows for the possibility of a shorter ‘second phase’ procedure on this side, and for the smaller volume stone burden to be cleared at a second phase bilateral procedure.
Postoperative management
The first question for postoperative management is whether the patient needs a JJ stent, and, if so, for how long should it remain in situ. In the situation of bilateral FURS, a minimum of one JJ stent should be used to avoid the risk of postoperative obstructive renal failure. In the case series on bilateral stones referenced in the perfect case section above, two patients who had undergone a bilateral procedure were left unstented. Both became anuric and were stented as emergency [3].
Similarly, patients who have an access sheath inserted ‘de novo’ (i.e. into a previously unstented ureter) are likely to have increased postoperative pain if unstented compared with patients who have been stented. However, patients with a prior stent undergoing FURS with an access sheath in situ can be left unstented if the procedure itself has been uneventful [10].
If a stent is inserted, the time it is left in situ should be minimised to reduce stent-related symptoms. In this regard, leaving the stent ‘on a string’ can facilitate earlier removal than might be achieved with arranging its extraction with a flexible cystoscope or in an outpatient setting. In a systematic review of eight studies, Oliver et al . showed no difference in pain scores or urinary symptoms between patients with a standard JJ stent versus one on strings, and confirmed a shorter dwell time for patients with strings left on. Furthermore, the majority of patients were able to remove their stents at home, thereby saving the cost of a journey back to hospital, as well as the cost of the procedure itself. The risk of this approach is inadvertent dislodgment and removal earlier than planned, which occurred in approximately 10% of patients. This technique is therefore not suitable for patients with a strong indication for a longer stent dwell, such as intraoperative ureteric trauma (as discussed in the section on access sheaths), concern for postoperative UTI, or in patients whose original stone burden requires a second phase procedure for residual stones [11].
“The first question for postoperative management is whether the patient needs a JJ stent; the second is how long it remains in situ to minimise stent-related symptoms.”
The next question relates to the possibility of residual fragments and the risk these may pose to a future episode of ureteric colic, or the need for further procedures to treat them. Chew and colleagues looked at the natural history of asymptomatic stone fragments left behind after ureteroscopy (URS) and found that only 56% of patients required no intervention and remained asymptomatic. Fifteen percent experienced complications that required no intervention but intervention was required in 29% of patients, with a greater risk associated with larger fragments. They concluded that complete stone-free status is the most effective strategy to reduce stone events following ureteroscopy. Fragments >4mm were associated with an increased risk of intervention, while fragments >2mm were more likely to grow over time but were not found to be associated with re-intervention of complications in this analysis [12].
Conclusion
Whilst FURS has held the position of ‘treatment of choice’ for renal calculi around 15mm in size, with ESWL used for stones less than 10mm and PCNL reserved for stones greater than 20mm, developments in miniaturising nephroscopes, allowing smaller, less invasive tracts to treat stones by percutaneous surgery, means that treatment choices need to be made on an individual basis. In the next and final article in the series, the role for PCNL, including its miniaturised versions, will be considered.