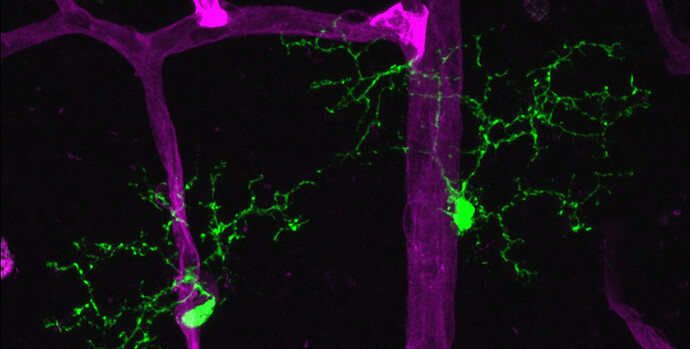
A team led by Harvard Medical School researchers at Massachusetts Eye and Ear has shown that microglia, the primary immune cells of the central nervous system—including the retina of the eye—serve as “gatekeepers,” or biosensors and facilitators, of neuroinflammation in a preclinical model of autoimmune uveitis.
Uveitis is one of the leading causes of blindness, accounting for approximately 10 percent of significant visual impairment worldwide.
Until now, the role of microglia in retinal disease has not been fully understood.
In a report published online in Proceedings of the National Academy of Sciences , the researchers describe, for the first time, a role for microglia in directing the initiation of autoimmune uveitis by orchestrating the inflammatory response within the retina.
In reaction to disease induction, microglia closely associate with the retinal vasculature and facilitate inflammatory immune cell entry past the blood-brain barrier, or ocular barrier, into the retina, the researchers found.
When the researchers depleted microglia in this model, they observed that the disease was completely blocked.
“Normally, the blood-brain barrier serves as an impediment and prevents the immune response from going into tissues of the central nervous system, including the retina,” said senior author Kip Connor, HMS associate professor of ophthalmology and a vision researcher at Mass. Eye and Ear.
“However, our results provide clear evidence that, in the context of uveitis, microglia can facilitate entry of inflammatory immune cells into the retina and enable the host immune responses to attack cells that are not normally recognized by the immune system,” he said.
“The gateway not only has implications for treating uveitis, but may provide future avenues for drug delivery across the blood-brain barrier for other diseases of the central nervous system, Connor said.
About uveitis
Despite significant advances in research and therapeutics, the prevalence of autoimmune uveitis has not been reduced in the past 30 years.
Uveitis is characterized as inflammation of the retina as well as the uveal tissues, optic nerve and vitreous, wherein a large influx of immune cells into the eye coincides with elevated inflammatory destruction.
Uveitis caused by an autoimmune disease, defined by autoreactive inflammation against the retina, occurs in a variety of diseases, including Bechet’s disease, sarcoidosis and Vogt-Koyanagi-Harada disease. Patients with autoimmune uveitis often suffer serious visual loss after persistent inflammation due to immune-mediated damage to the neuronal cells of the retina.
Since microglia have multiple phenotypes and different stages of activation that can be associated with either harmful or beneficial effects in disease pathogenesis, their role and function in disease progression is not well defined.
Harnessing insights
Researchers across all fields of medicine have recently begun to elucidate the function of microglial cells in various conditions.
For example, in Alzheimer’s, Parkinson’s and other neurodegenerative diseases of the brain, microglia are thought to be harmful.
In ophthalmology, it is known that microglial cells are activated in response to a number of developmental and disease indications, and their roles in disease are thought to be context dependent, where they can be either beneficial or harmful.
“These findings provide the first insights into how microglia respond and function during a systemic autoimmune disease targeting the eye,” said lead author Yoko Okunuki, HMS instructor of ophthalmology and an investigator in Connor’s laboratory.
“It is our hope that these findings can be harnessed for future targeted therapies for autoimmune uveitis,” said Joan Miller, the HMS David Glendenning Cogan Professor of Ophthalmology at Mass. Eye and Ear and chair of the Department of Ophthalmology at HMS.
“It is becoming increasingly clear that microglia are involved in a number of central nervous system-related autoimmune diseases, so these results are of interest to the broader scientific community as well,” added Miller, who is also chief of ophthalmology at Mass. Eye and Ear, Massachusetts General Hospital and Brigham and Women’s Hospital.
Additional authors include Ryo Mukai, Takeshi Nakao, Steven J. Tabor, Reza Dana and Bruce R. Ksander of HMS and Mass. Eye and Ear and Oleg Butovsky of HMS and Brigham and Women’s.
This research was supported by the National Eye Institute of the National Institutes of Health (grant R01EY027303), the Massachusetts Lions Eye Research Fund and an American Macular Degeneration Foundation prevention award.