Disulfide bonds play a crucial role in the structure and function of immunoglobulins, also known as antibodies.
Immunoglobulins are proteins produced by B cells in the immune system and are a key component of the body’s defense against pathogens like bacteria, viruses, and other foreign invaders.
Disulfide bonds are covalent bonds formed between two sulfur atoms from the amino acids cysteine, and they are essential for the proper functioning of immunoglobulins in several ways:
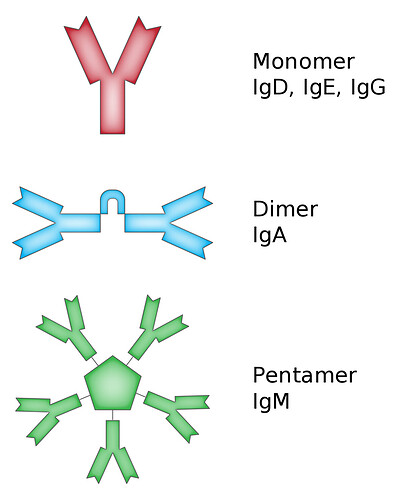
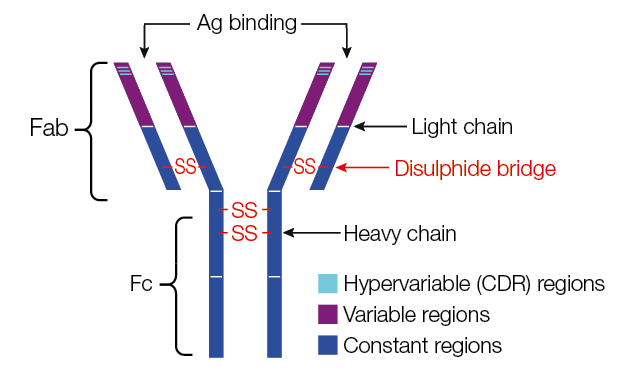
- Stabilizing the Protein Structure: Disulfide bonds help stabilize the three-dimensional structure of immunoglobulins. Immunoglobulins have a Y-shaped structure consisting of two heavy chains and two light chains. Disulfide bonds form bridges between different parts of the chains, holding them together and maintaining the correct shape of the antibody molecule.
- Maintaining Antigen-Binding Sites: The tips of the Y-shaped immunoglobulin molecule contain antigen-binding sites, which are highly specific for recognizing and binding to antigens (foreign substances) such as pathogens. Disulfide bonds help maintain the structural integrity of these antigen-binding sites, ensuring that they can bind to antigens with high affinity and specificity.
-
Forming Dimers or Multimers: Some antibodies, particularly IgA and IgM, exist in forms with multiple antibody units joined together by disulfide bonds. IgA antibodies can form dimers (two antibody units) or even larger structures, while IgM antibodies typically exist as pentamers or hexamers. These multimeric structures enhance the antibodies’ ability to trap and neutralize pathogens, as they can bind to multiple antigens simultaneously.
-
Enhancing Stability in the Extracellular Environment: Immunoglobulins often function outside the cells in the bloodstream and tissues. Disulfide bonds contribute to the stability of antibodies in the extracellular environment by preventing them from unfolding or denaturing due to environmental factors like changes in pH or temperature.
-
Facilitating Effector Functions: Immunoglobulins play a role in immune defense not only by binding to antigens but also by engaging with immune system components. Disulfide bonds are involved in the Fc region of the antibody, which interacts with various immune cells and molecules, including phagocytes and complement proteins. These interactions help mediate effector functions, such as opsonization (marking pathogens for destruction), complement activation, and antibody-dependent cellular cytotoxicity (ADCC).
In summary, disulfide bonds in immunoglobulins are essential for maintaining the structural integrity, antigen-binding specificity, and functional versatility of these antibodies. They contribute to the effectiveness of the immune response by allowing antibodies to recognize and neutralize a wide range of pathogens and by mediating interactions with other immune system components.